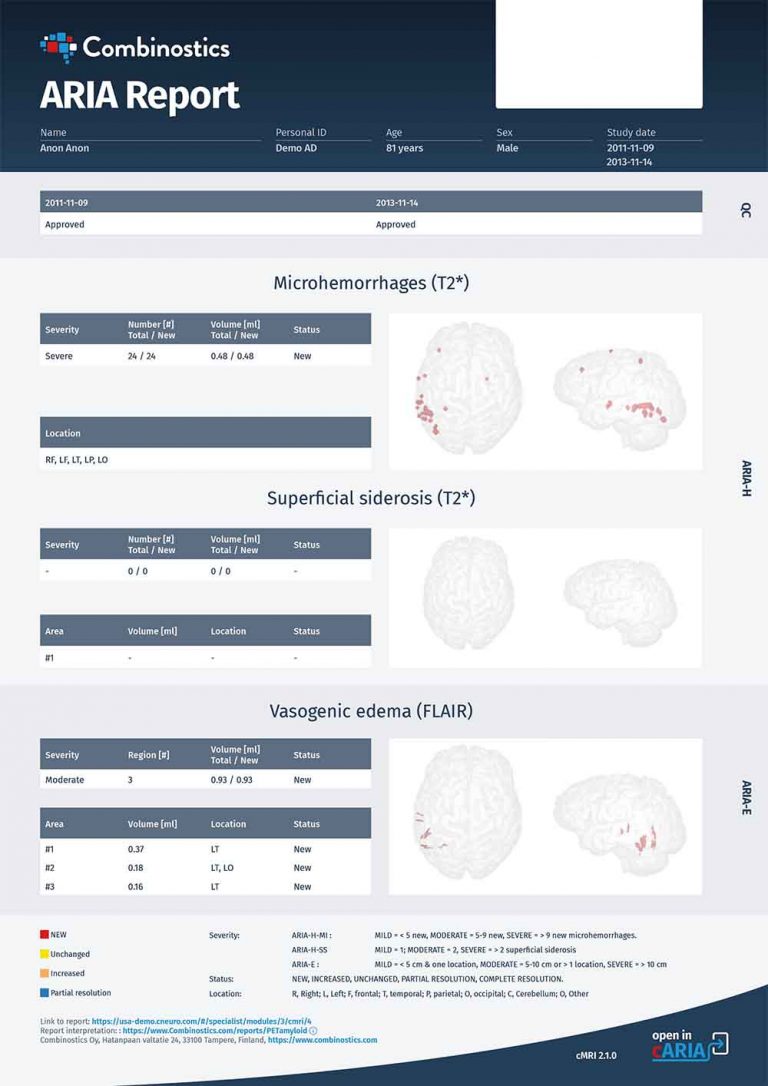
With Alzheimer’s disease (AD) contributing to 60%-70% of the 55 million people living with dementia worldwide, the quest for solutions that slow, or even stop, the underlying disease processes has been a long, sometimes arduous, journey. Beyond the traditional lifestyle recommendations such as physical activity, a healthy weight, and avoiding smoking, people at risk of AD have had few pharmaceutical options until the approval by the US FDA of aducanumab (ADUHELM™) in mid-2021 and now the approval of lecanemab (LEQEMBI™) earlier this month. Both are disease-modifying drugs (DMDs) targeting amyloid beta plaques in the brain, a hallmark of AD.
- helped address the most critical need for patients with AD (DMDs that slow cognitive and functional decline)
- supported the amyloid hypothesis
- opened the gate for other therapies to be approved based on biomarker endpoints (e.g., decreased amyloid levels in the brain)
- provided hope for people with AD and their caregivers
AD management is benefitting from broad development.
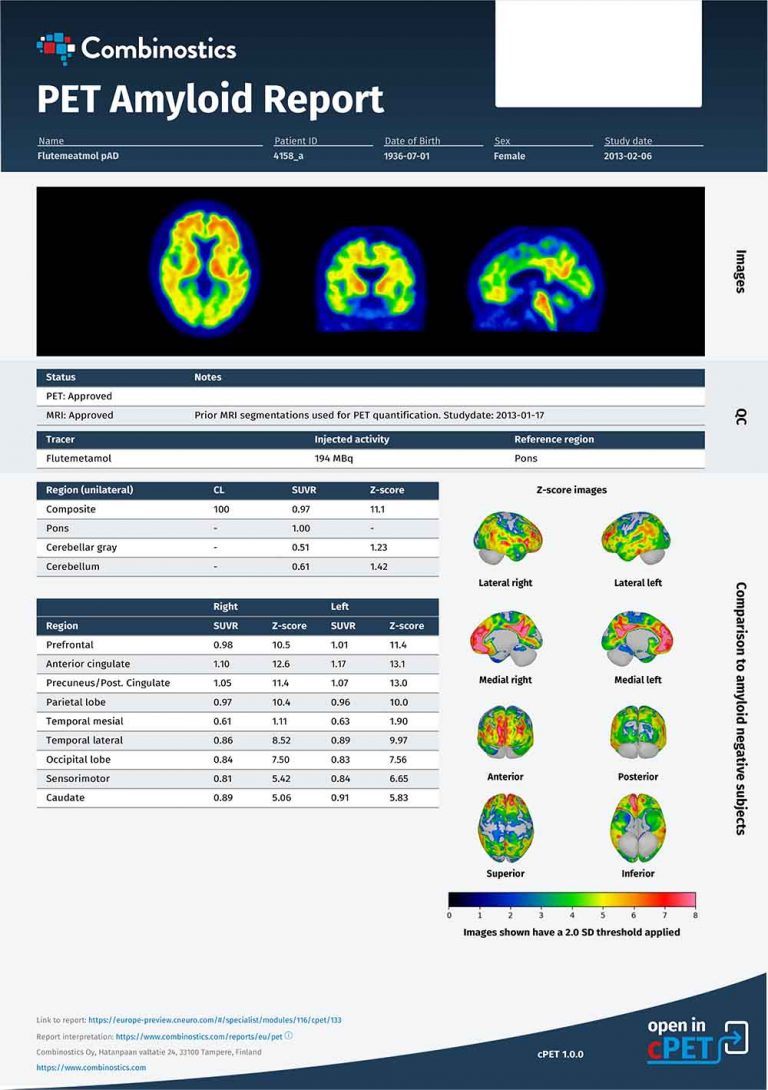
One thing is certain — DMDs will be part of the future of AD management, and the entire patient pathway for DMD in AD requires due diligence for the maximum benefit with the lowest risk. This pathway encompasses early disease detection, identification of eligible patients, and monitoring of both efficacy and side effects. Biomarkers, including imaging biomarkers, will play an important part in this pathway, especially to overcome lingering skepticism about Aduhelm’s efficacy and concerns about its safety profile.
Detect AD as soon as possible.
Identify appropriate, eligible patients.
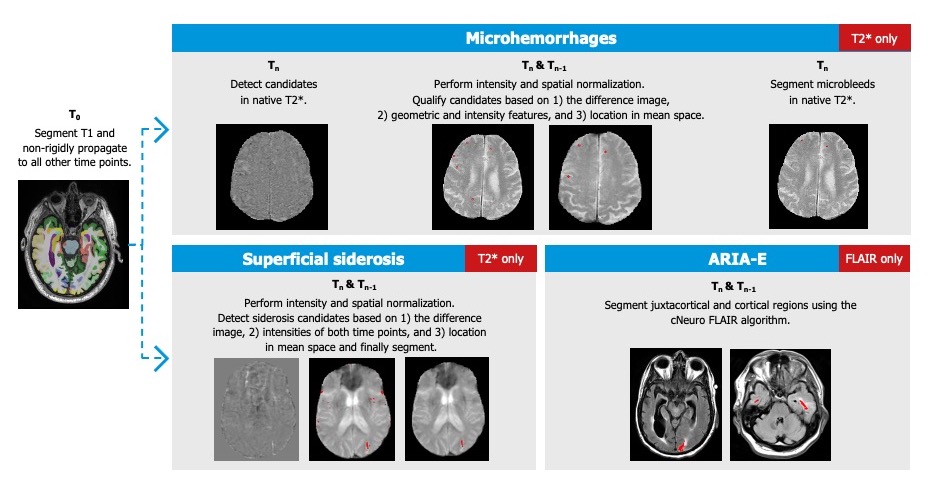
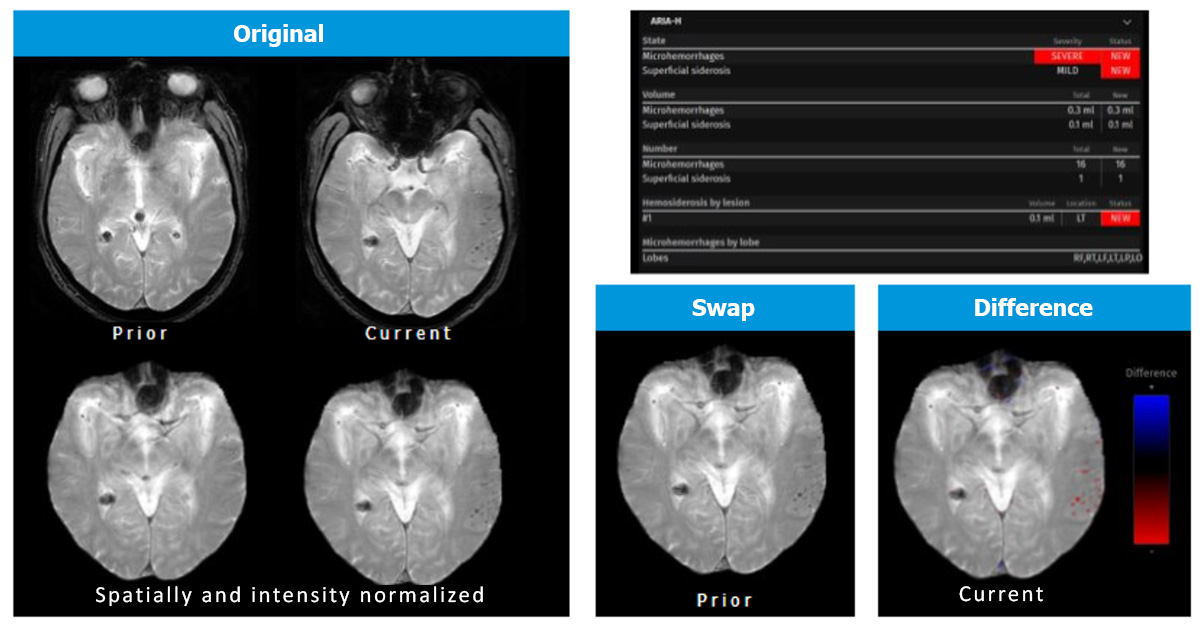
A combination of biological mechanisms might be the cause of both ARIA-E and ARIA-H during amyloid-modifying treatment: increased cerebrovascular permeability due to increased clearance of β-amyloid neuritic plaques, a resulting saturation of the perivascular drainage, interaction between the antibodies and deposits of vascular amyloid, and a weakening of the vessel wall.
Characteristic | ARIA-E | ARIA-H |
|---|---|---|
Primary MRI features
|
|
|
Nature of leakage products | Proteinaceous fluids | Blood degradation products (i.e., hemosiderin) |
Location of increased vascular permeability |
|
|
Adapted from Filippi et al.
ARIA-E
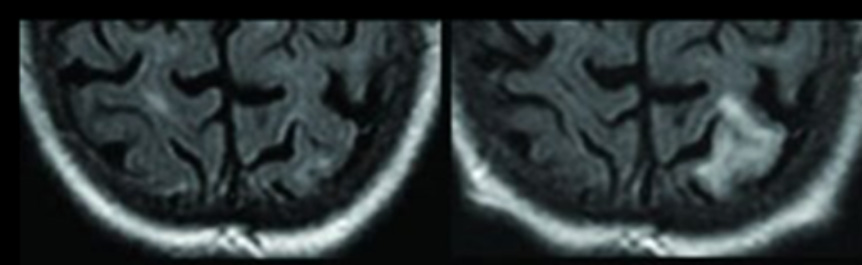
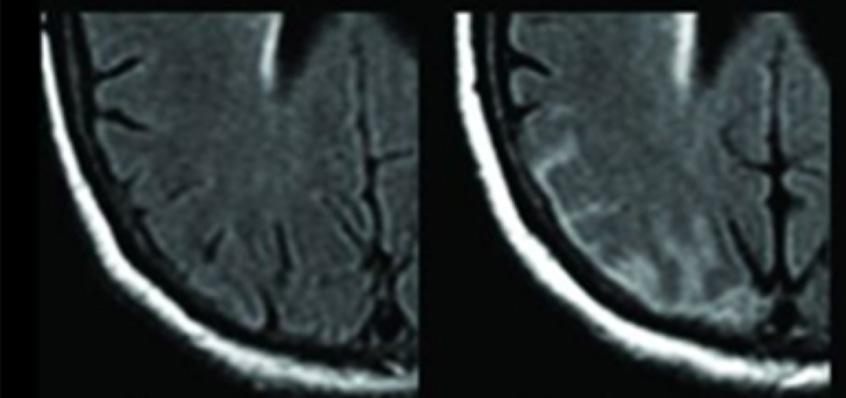
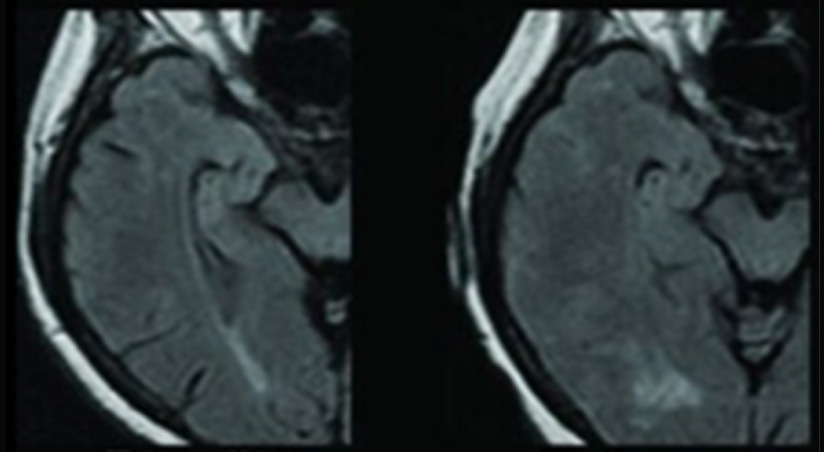
ARIA-E tends to be dose-dependent, with a greater risk of occurrence at higher doses of amyloid-targeting therapies. In addition, being an ApoE ε4 carrier has emerged as an important risk factor for treatment-related ARIA-E, potentially related to the associated higher parenchymal and vascular β-amyloid load. In these patients, anti-β-amyloid therapies could result in larger clearance, greater vascular permeability, and therefore easier extravasation of fluid (ARIA-E) and erythrocytes (ARIA-H).
ARIA-H
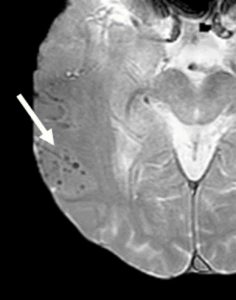
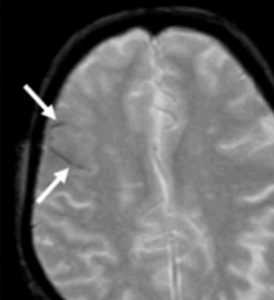
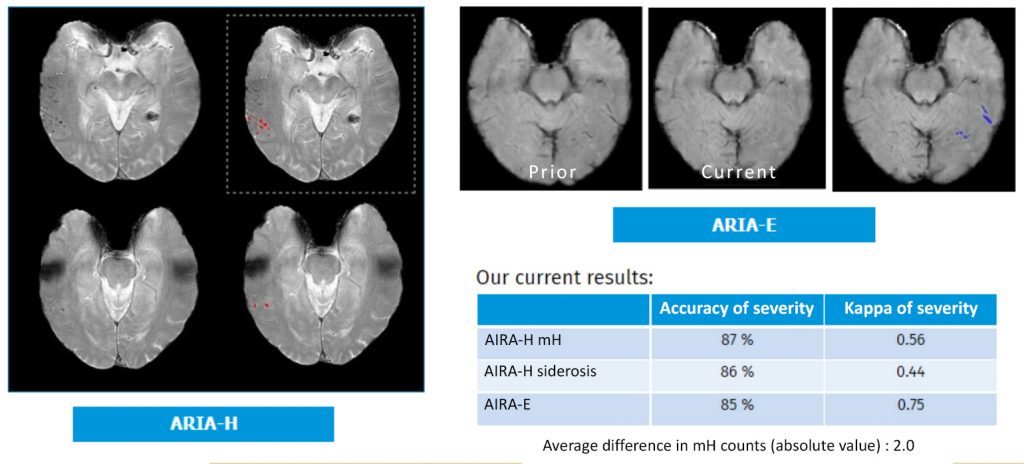
Size criteria have also been recommended, at cut-offs of ≤10 mm or ≤5 mm diameter. However, the technical features of image acquisition create challenges for consistently measuring the size of microhemorrhages. In addition, a “blooming” effect can occur: a phenomenon in which the apparent size of a microhemorrhage on MRI is larger than the size of the histologically defined hemosiderin deposit. This phenomenon is related to signal loss that extends spatially beyond the area of the deposit.
Closely monitor efficacy and safety.
- Were mostly mild to moderate (91%), based on central imaging reads
- Were asymptomatic (78%)
- Occurred during the first 3 months of treatment (71%)
- Resolved within 4 months after detection (81%)
- Field strength of at least 1.5 T
- Acquisition of GRE and/or FLAIR sequences
- Section thickness of 5 mm
- Echo time of 20 milliseconds