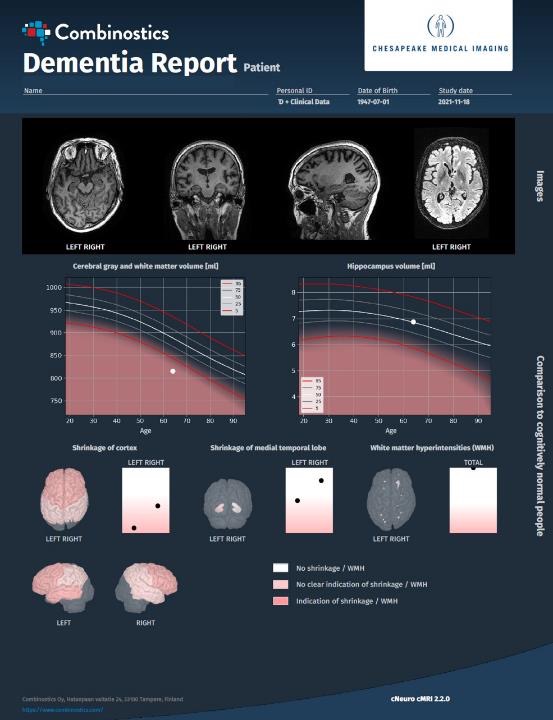
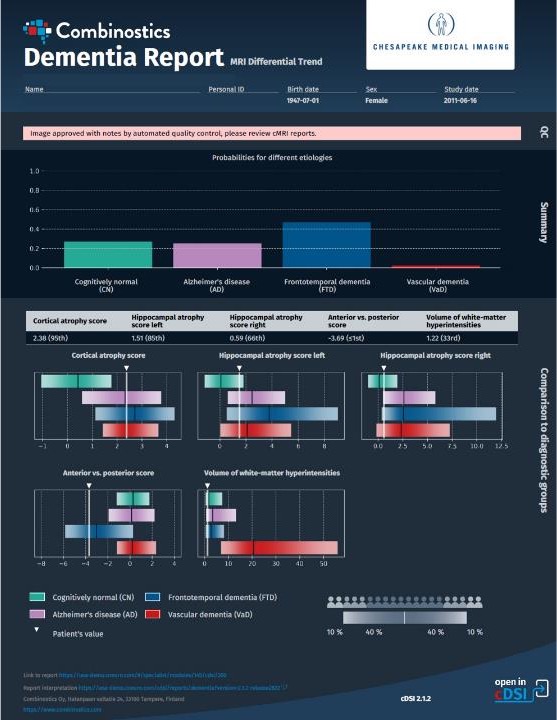
One benefit of expanding our reach in radiology practices globally is the opportunity to work with pioneers in the field, hearing about their experiences and learning about how we’re meeting specific pain points with our products and what we can do to make our applications even more effective for our end users. We recently spoke with Kevin Berger, MD, a radiologist specializing in diagnostic radiology and neuroradiology at Chesapeake Medical Imaging. He has a long history of contributing to the development of imaging modalities such as PET and MRI, including as a consultant to the U.S. National Institutes of Health, as well as quantification tools. He recently started using our cMRI™ application and is excited about cMRI’s approach to quantifying patient images.
What sparked Dr. Berger’s interest in neuroradiology?
He was instrumental in developing a software program using PET to diagnose dementias, which later was licensed and became available commercially. Since then, he’s advised on the development of other similar programs using PET or MRI, has contributed as the lead clinical site for the validation of imaging software programs, and has assisted companies with obtaining regulatory clearance for their software programs to assess neurodegenerative diseases. As he said, he knows firsthand that “for imaging quantification software, the timeline from research application to clinical use can be long.”
Have quantitative imaging programs become more accepted by radiologists?
When can quantitative imaging assessments have the greatest impact?
What role does cMRI have in Dr. Berger’s practice?
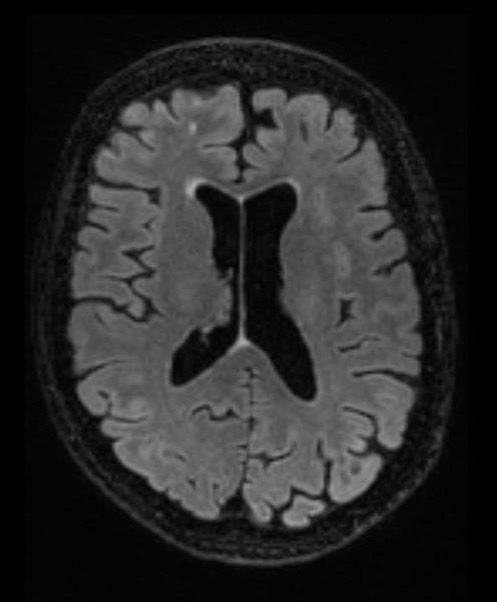
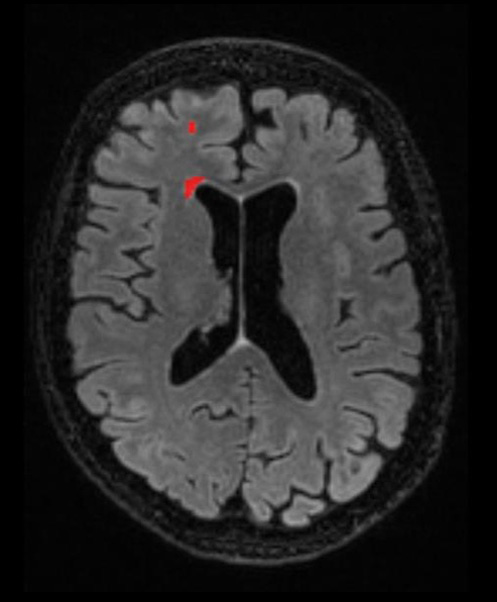
The end goal is a more holistic approach to patient assessment.
We’re looking forward to continuing to work with Dr. Berger and benefit from his insights on radiology practice, imaging software, and more. If you’d like to learn more about cMRI, contact us for a discussion or a demo, or take advantage of our free 10-day trial of cMRI on your own patient cases.