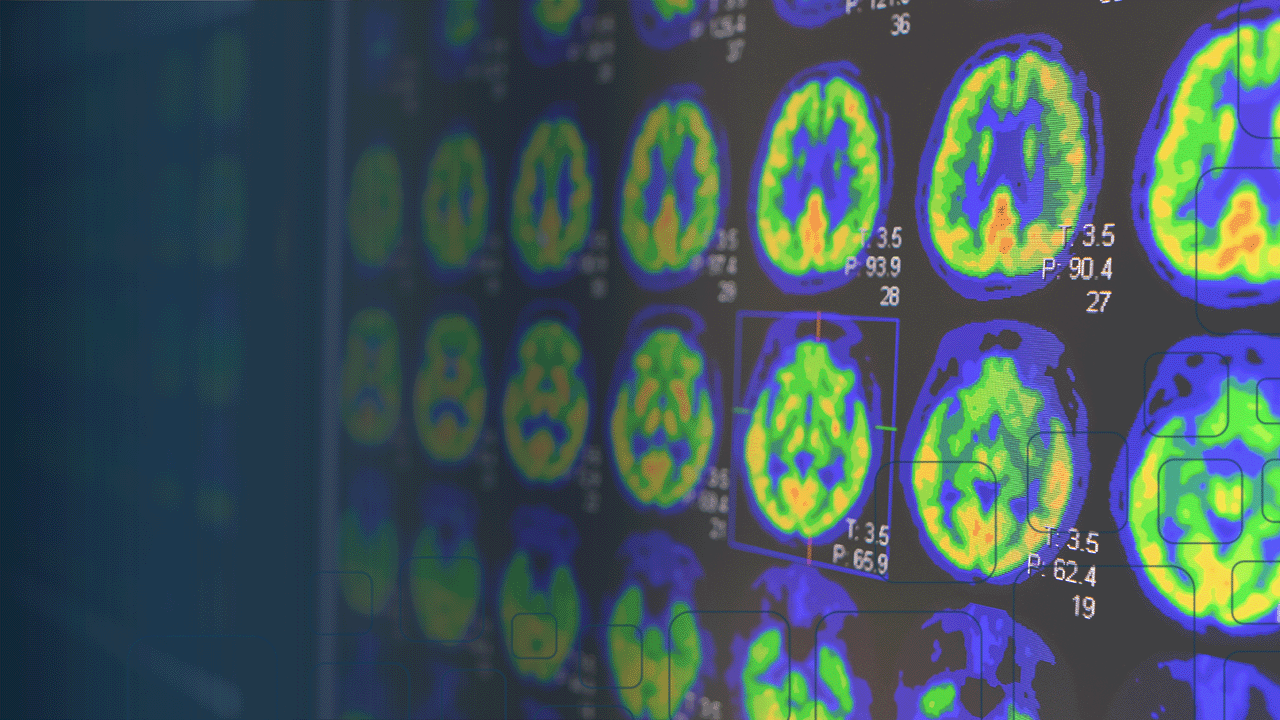
Company launch cPET™ solution for automated amyloid and FDG brain PET quantification in the United States.
Tampere, Finland, September 26, 2023 — Combinostics, the only company providing a complete imaging and decision support solution spanning the entire patient care pathway for the early detection, diagnosis, and ongoing management of major neurological disorders, today announced that it has obtained FDA 510(k) clearance for its cNEURO cPET application.
cPET’s unique image registration technique allows for increased accuracy and confidence with precise quantification of FDG and amyloid PET scans with comparison to tracer-specific normative data. cPET employs both a PET-only and a PET-MRI workflow. If the patient’s MRI is available, cPET integrates segmentations from the company’s cMRI application to provide increased accuracy in the quantification of brain regions including small structures such as the hippocampus.
“cPET further complements our suite of holistic solutions for neurological disorders,” said Lennart Thurfjell, CEO of Combinostics. “We are delighted to be able to make cPET available to our customers in the United States and continue to build our ability to support the entire patient care pathway from early detection, to diagnosis, and ongoing management of major neurological disorders”.
For more information or to view a demo of Combinostics’ solutions, schedule a demo.
About Combinostics
Combinostics’ AI-powered cNeuro suite of products helps clinicians make a difference in the lives of patients with neurological disorders. By quantifying brain images and integrating patient data from multiple sources with insights from previous patients, the company’s unique software tools provide radiologists and clinicians the support they need for confident, evidence-based diagnostic and management decisions. The company was founded in 2014 and is headquartered in Tampere, Finland. For more information, please visit www.combinostics.com.