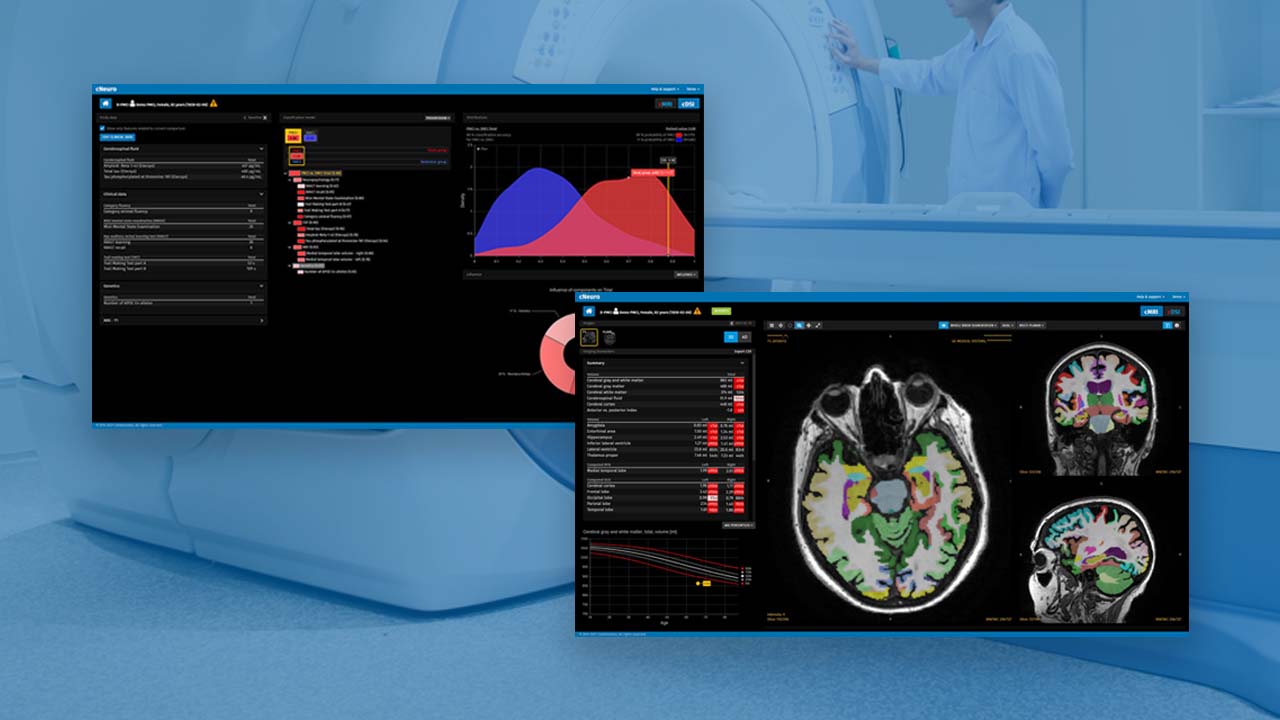
Certification ensures that the safety and performance of the cDSI and cMRI applications meet the European Union’s updated medical device regulatory framework
Tampere, Finland, June 21, 2022—Combinostics, the only company providing a complete solution from early detection and diagnosis to the ongoing management of neurological disorders, today announced that its cDSI™ and cMRI™ applications, part of the the cNeuro® platform, have received EC-certification under the European Union (EU)’s Medical Device Regulation (MDR).
“With the EC-certification of cDSI and cMRI under MDR, we can continuously deliver our services to our customers in the EU, United Kingdom, and Switzerland,” said Richard Hausmann, CEO of Combinostics. “The certification is another example of our commitment to supporting radiologists and neurologists in the diagnosis and ongoing care of patients with neurodegenerative disorders.”
The more comprehensive MDR replaces the previous Medical Device Directive (MDD) and further ensures that the safety and performance of the cDSI and cMRI applications meet the regulations set forth by the EU. Additionally, MDR certification guarantees that the applications comply with all applicable CE-marking regulations. All medical devices with European market access must be certified under this new regulatory framework and have an EU declaration of conformity.
The artificial intelligence (AI)–powered cNeuro platform offers tools and software to support clinicians in the early detection, differentiation of diagnosis, treatment, and ongoing management of patients with neurodegenerative disorders, including Alzheimer’s disease, dementia, multiple sclerosis, traumatic brain injuries, and epilepsy. The cMRI application within cNeuro supports radiologists with AI-enabled imaging quantification, while the cDSI application combines and analyzes key patient and demographic data to support neurologists in diagnosis, testing decisions, prediction of disease trajectory, and treatment management.
For more information or to view a demo of the entire cNeuro suite, join the team at booth AI-09 at the European Congress of Radiology (ECR), July 13-17, 2022, in Vienna, Austria. Schedule an in-person or virtual meeting at combinostics.com/ecr.
Combinostics at ECR 2022
Booth: AI-09
July 13-17, 2022
Austria Center Vienna (ACV)
Bruno Kreisky-Platz 1, 1220 Vienna, Austria
myesr.org
About Combinostics
Combinostics’ AI-powered cNeuro suite of products helps clinicians make a difference in the lives of patients with neurological disorders. By quantifying brain images and integrating patient data from multiple sources with insights from previous patients, the company’s unique software tools provide radiologists and clinicians the support they need for confident, evidence-based diagnostic and management decisions. The company was founded in 2014 and is headquartered in Tampere, Finland. For more information, please visit combinostics.com.